- Phone +91 7989696762, +91 9346911718
- E-mail contact@htsbiopharma.com





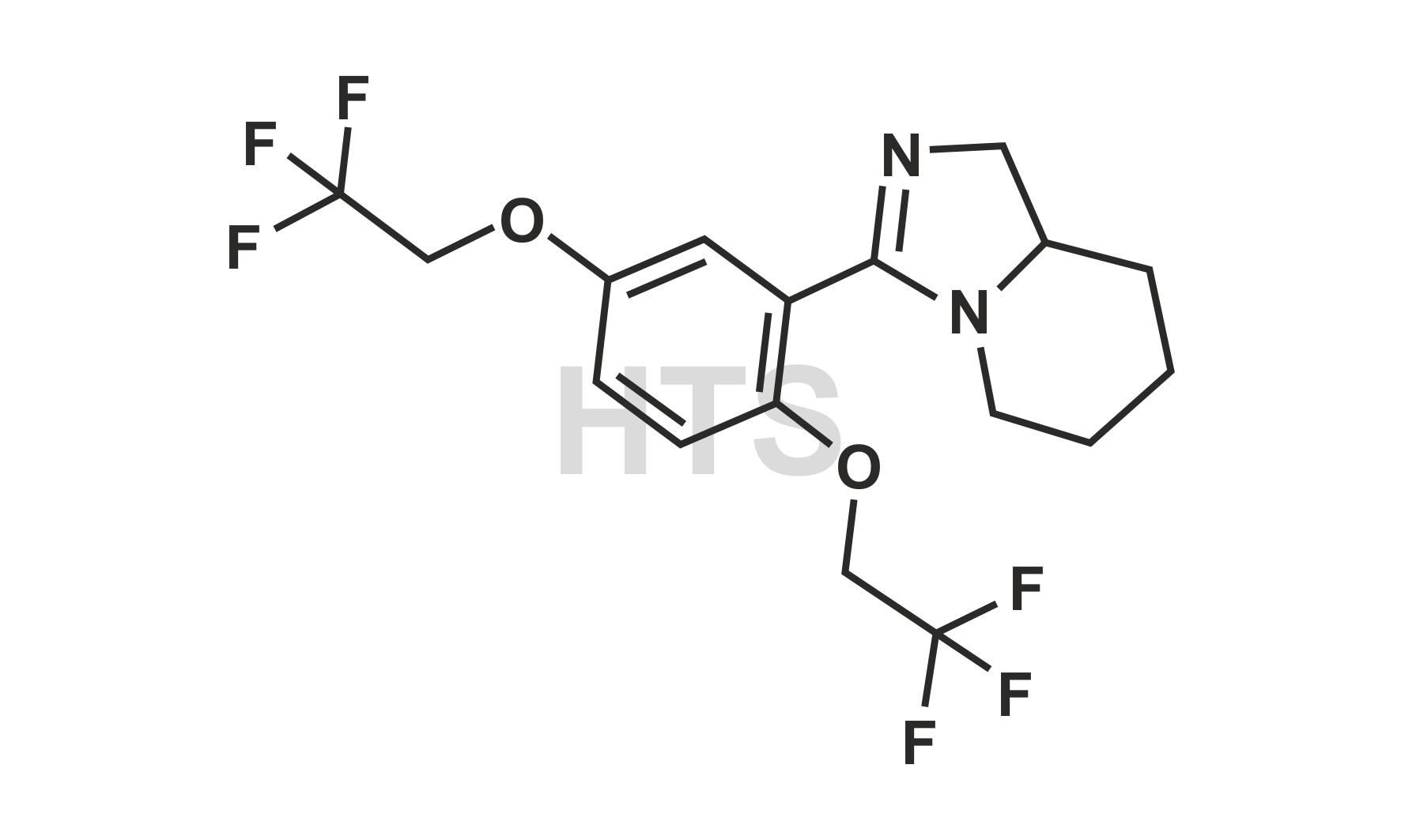
CAT No:
HTS-F-017A
CAS No :
NA
Mol. Formula:
C17H18F6N2O2
Mol. Weight:
396.33
Inv. Status:
In Stock
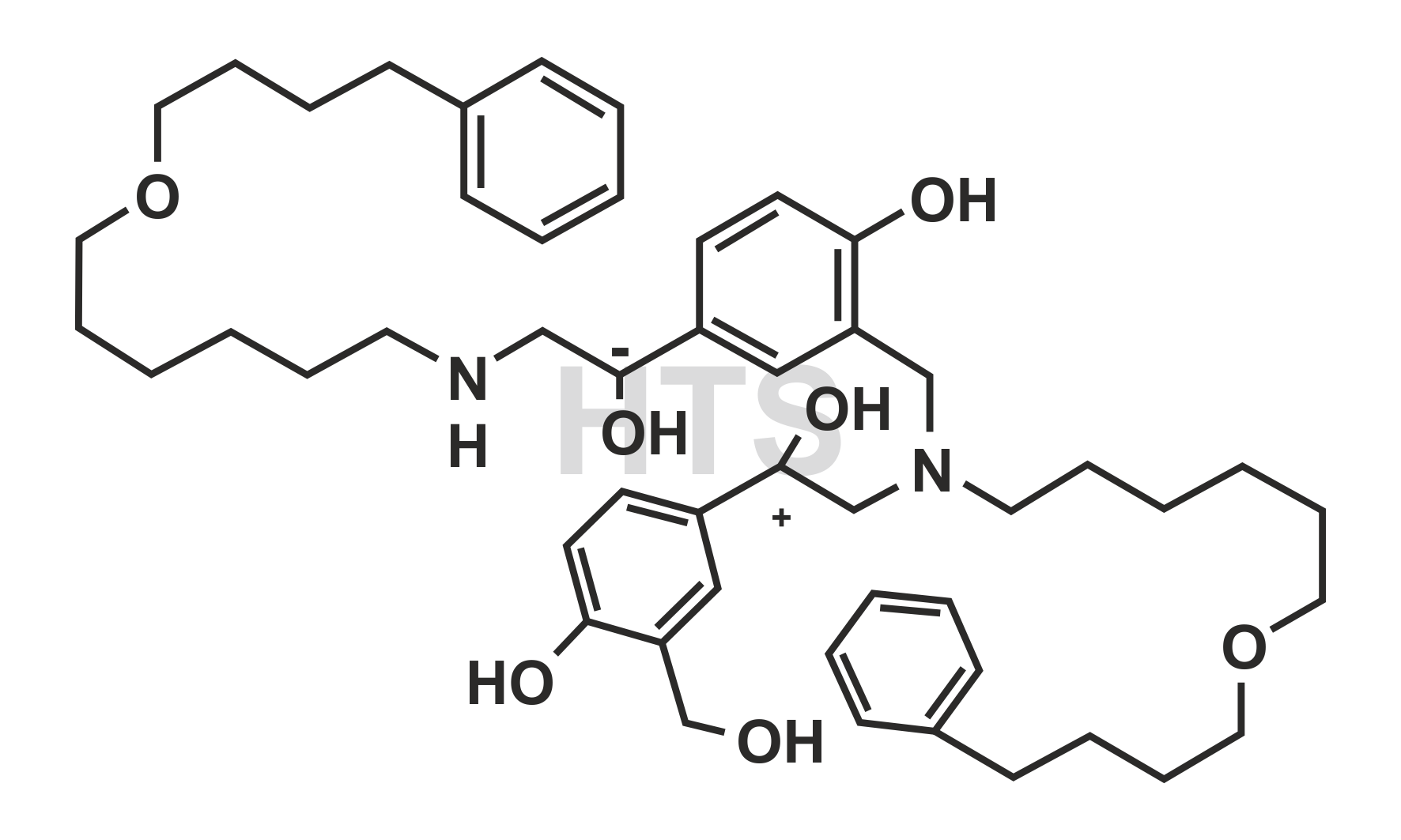
CAT No:
HTS-S-001G
CAS No :
1391051-88-9
Mol. Formula:
C50H72N2O7
Mol. Weight:
813.13
Inv. Status:
In Stock
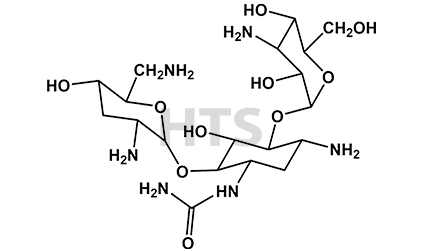
CAT No:
HTS-T-027F
CAS No :
NA
Mol. Formula:
C19H38N6O10
Mol. Weight:
510.5
Inv. Status:
In Stock
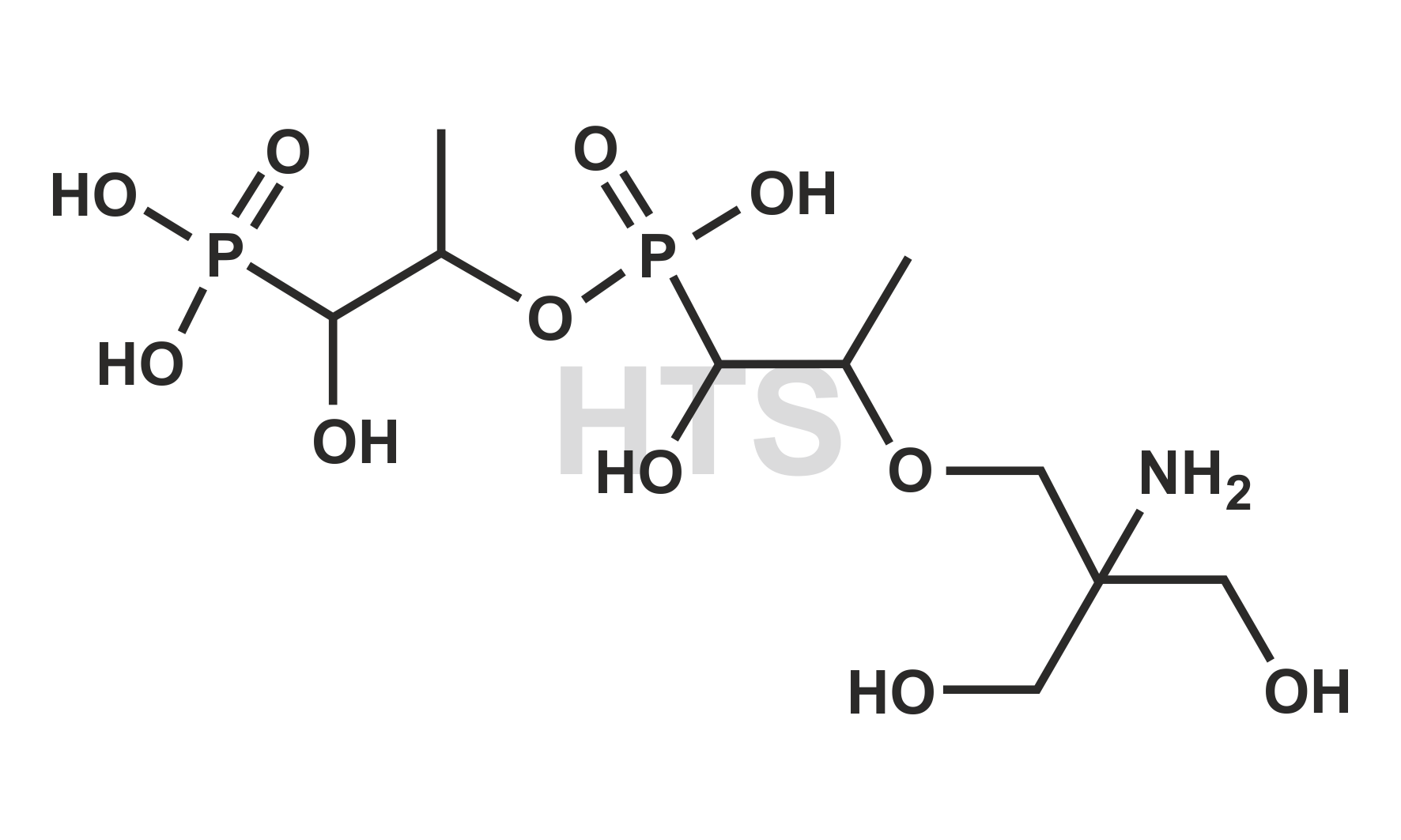
CAT No:
HTS-F-014D
CAS No :
1262243-12-8
Mol. Formula:
C10H25NO11P2
Mol. Weight:
397.25
Inv. Status:
In Stock
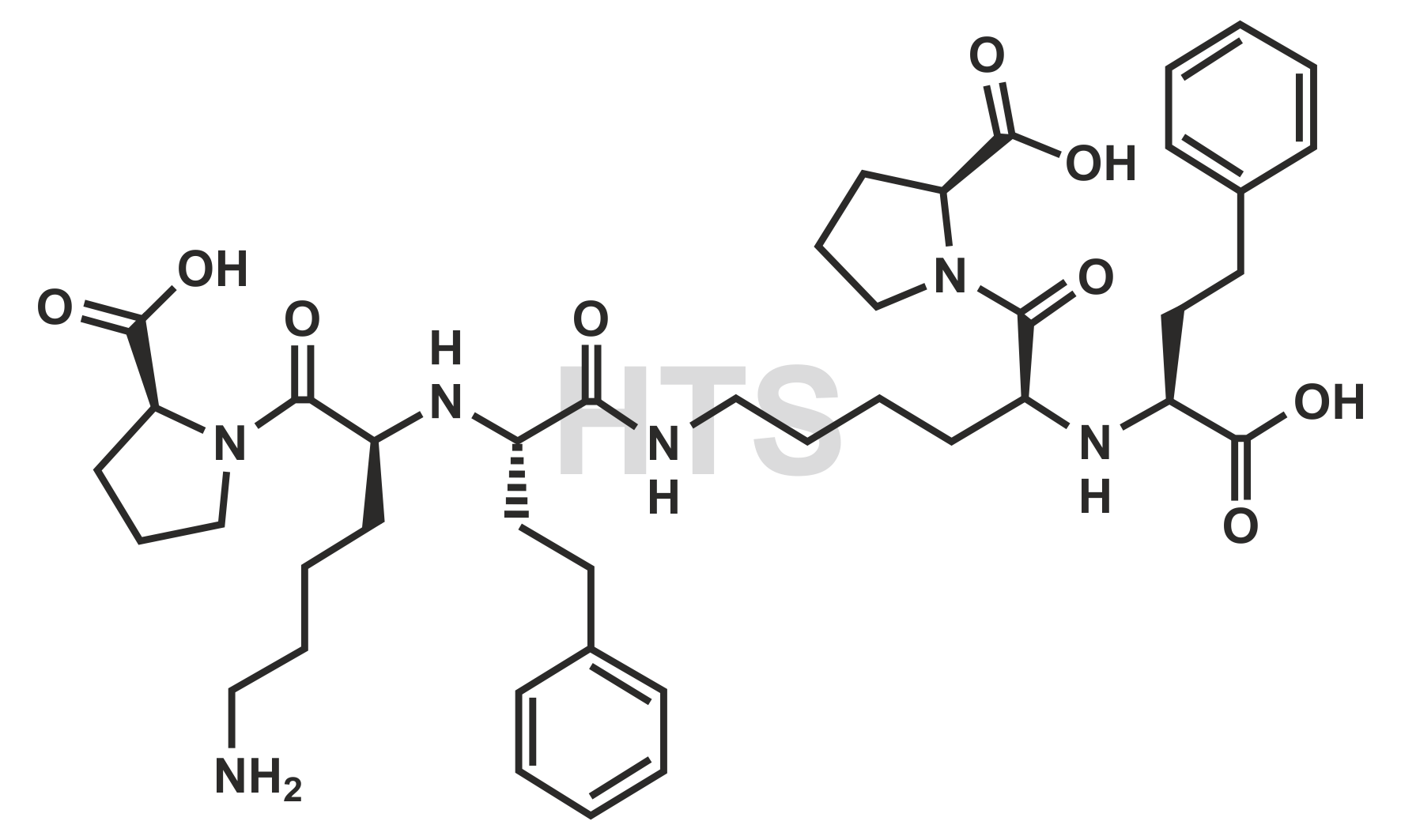
CAT No:
HTS-L-008G
CAS No :
1356839-89-8
Mol. Formula:
C42H60N6O9
Mol. Weight:
792.98
Inv. Status:
In Stock
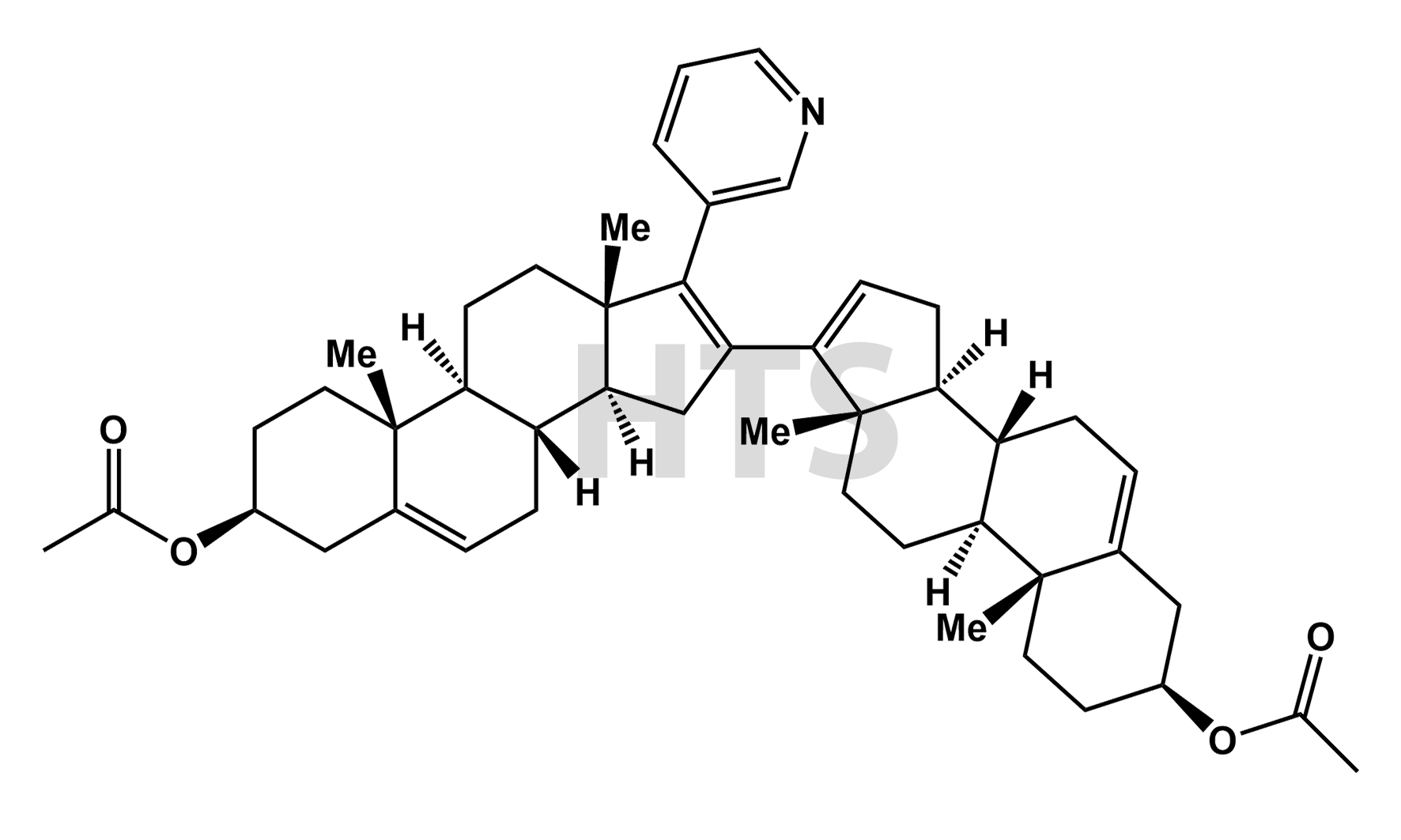
CAT No:
HTS-A-010AAD
CAS No :
186826-68-6
Mol. Formula:
C47H61NO4
Mol. Weight:
704.01
Inv. Status:
In Stock
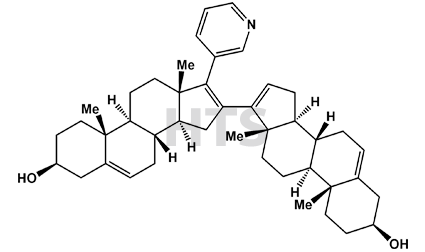
CAT No:
HTS-A-010AD
CAS No :
186826-70-0
Mol. Formula:
C43H57NO2
Mol. Weight:
619.93
Inv. Status:
In Stock

CAT No:
HTS-F-017A
CAS No :
NA
Mol. Formula:
C17H18F6N2O2
Mol. Weight:
396.33
Inv. Status:
In Stock

CAT No:
HTS-S-001G
CAS No :
1391051-88-9
Mol. Formula:
C50H72N2O7
Mol. Weight:
813.13
Inv. Status:
In Stock

CAT No:
HTS-T-027F
CAS No :
NA
Mol. Formula:
C19H38N6O10
Mol. Weight:
510.5
Inv. Status:
In Stock

CAT No:
HTS-F-014D
CAS No :
1262243-12-8
Mol. Formula:
C10H25NO11P2
Mol. Weight:
397.25
Inv. Status:
In Stock

CAT No:
HTS-L-008G
CAS No :
1356839-89-8
Mol. Formula:
C42H60N6O9
Mol. Weight:
792.98
Inv. Status:
In Stock

CAT No:
HTS-A-010AAD
CAS No :
186826-68-6
Mol. Formula:
C47H61NO4
Mol. Weight:
704.01
Inv. Status:
In Stock

CAT No:
HTS-A-010AD
CAS No :
186826-70-0
Mol. Formula:
C43H57NO2
Mol. Weight:
619.93
Inv. Status:
In Stock

CAT No:
HTS-F-017A
CAS No :
NA
Mol. Formula:
C17H18F6N2O2
Mol. Weight:
396.33
Inv. Status:
In Stock

CAT No:
HTS-S-001G
CAS No :
1391051-88-9
Mol. Formula:
C50H72N2O7
Mol. Weight:
813.13
Inv. Status:
In Stock

CAT No:
HTS-T-027F
CAS No :
NA
Mol. Formula:
C19H38N6O10
Mol. Weight:
510.5
Inv. Status:
In Stock

CAT No:
HTS-F-014D
CAS No :
1262243-12-8
Mol. Formula:
C10H25NO11P2
Mol. Weight:
397.25
Inv. Status:
In Stock

CAT No:
HTS-L-008G
CAS No :
1356839-89-8
Mol. Formula:
C42H60N6O9
Mol. Weight:
792.98
Inv. Status:
In Stock

CAT No:
HTS-A-010AAD
CAS No :
186826-68-6
Mol. Formula:
C47H61NO4
Mol. Weight:
704.01
Inv. Status:
In Stock

CAT No:
HTS-A-010AD
CAS No :
186826-70-0
Mol. Formula:
C43H57NO2
Mol. Weight:
619.93
Inv. Status:
In Stock

CAT No:
HTS-F-017A
CAS No :
NA
Mol. Formula:
C17H18F6N2O2
Mol. Weight:
396.33
Inv. Status:
In Stock

CAT No:
HTS-S-001G
CAS No :
1391051-88-9
Mol. Formula:
C50H72N2O7
Mol. Weight:
813.13
Inv. Status:
In Stock

CAT No:
HTS-T-027F
CAS No :
NA
Mol. Formula:
C19H38N6O10
Mol. Weight:
510.5
Inv. Status:
In Stock

CAT No:
HTS-F-014D
CAS No :
1262243-12-8
Mol. Formula:
C10H25NO11P2
Mol. Weight:
397.25
Inv. Status:
In Stock

CAT No:
HTS-L-008G
CAS No :
1356839-89-8
Mol. Formula:
C42H60N6O9
Mol. Weight:
792.98
Inv. Status:
In Stock

CAT No:
HTS-A-010AAD
CAS No :
186826-68-6
Mol. Formula:
C47H61NO4
Mol. Weight:
704.01
Inv. Status:
In Stock

CAT No:
HTS-A-010AD
CAS No :
186826-70-0
Mol. Formula:
C43H57NO2
Mol. Weight:
619.93
Inv. Status:
In Stock

CAT No:
HTS-F-017A
CAS No :
NA
Mol. Formula:
C17H18F6N2O2
Mol. Weight:
396.33
Inv. Status:
In Stock

CAT No:
HTS-S-001G
CAS No :
1391051-88-9
Mol. Formula:
C50H72N2O7
Mol. Weight:
813.13
Inv. Status:
In Stock

CAT No:
HTS-T-027F
CAS No :
NA
Mol. Formula:
C19H38N6O10
Mol. Weight:
510.5
Inv. Status:
In Stock

CAT No:
HTS-F-014D
CAS No :
1262243-12-8
Mol. Formula:
C10H25NO11P2
Mol. Weight:
397.25
Inv. Status:
In Stock

CAT No:
HTS-L-008G
CAS No :
1356839-89-8
Mol. Formula:
C42H60N6O9
Mol. Weight:
792.98
Inv. Status:
In Stock

CAT No:
HTS-A-010AAD
CAS No :
186826-68-6
Mol. Formula:
C47H61NO4
Mol. Weight:
704.01
Inv. Status:
In Stock

CAT No:
HTS-A-010AD
CAS No :
186826-70-0
Mol. Formula:
C43H57NO2
Mol. Weight:
619.93
Inv. Status:
In Stock

CAT No:
HTS-F-017A
CAS No :
NA
Mol. Formula:
C17H18F6N2O2
Mol. Weight:
396.33
Inv. Status:
In Stock

CAT No:
HTS-S-001G
CAS No :
1391051-88-9
Mol. Formula:
C50H72N2O7
Mol. Weight:
813.13
Inv. Status:
In Stock

CAT No:
HTS-T-027F
CAS No :
NA
Mol. Formula:
C19H38N6O10
Mol. Weight:
510.5
Inv. Status:
In Stock

CAT No:
HTS-F-014D
CAS No :
1262243-12-8
Mol. Formula:
C10H25NO11P2
Mol. Weight:
397.25
Inv. Status:
In Stock

CAT No:
HTS-L-008G
CAS No :
1356839-89-8
Mol. Formula:
C42H60N6O9
Mol. Weight:
792.98
Inv. Status:
In Stock

CAT No:
HTS-A-010AAD
CAS No :
186826-68-6
Mol. Formula:
C47H61NO4
Mol. Weight:
704.01
Inv. Status:
In Stock

CAT No:
HTS-A-010AD
CAS No :
186826-70-0
Mol. Formula:
C43H57NO2
Mol. Weight:
619.93
Inv. Status:
In Stock

CAT No:
HTS-F-017A
CAS No :
NA
Mol. Formula:
C17H18F6N2O2
Mol. Weight:
396.33
Inv. Status:
In Stock

CAT No:
HTS-S-001G
CAS No :
1391051-88-9
Mol. Formula:
C50H72N2O7
Mol. Weight:
813.13
Inv. Status:
In Stock

CAT No:
HTS-T-027F
CAS No :
NA
Mol. Formula:
C19H38N6O10
Mol. Weight:
510.5
Inv. Status:
In Stock

CAT No:
HTS-F-014D
CAS No :
1262243-12-8
Mol. Formula:
C10H25NO11P2
Mol. Weight:
397.25
Inv. Status:
In Stock

CAT No:
HTS-L-008G
CAS No :
1356839-89-8
Mol. Formula:
C42H60N6O9
Mol. Weight:
792.98
Inv. Status:
In Stock

CAT No:
HTS-A-010AAD
CAS No :
186826-68-6
Mol. Formula:
C47H61NO4
Mol. Weight:
704.01
Inv. Status:
In Stock

CAT No:
HTS-A-010AD
CAS No :
186826-70-0
Mol. Formula:
C43H57NO2
Mol. Weight:
619.93
Inv. Status:
In Stock

CAT No:
HTS-F-017A
CAS No :
NA
Mol. Formula:
C17H18F6N2O2
Mol. Weight:
396.33
Inv. Status:
In Stock

CAT No:
HTS-S-001G
CAS No :
1391051-88-9
Mol. Formula:
C50H72N2O7
Mol. Weight:
813.13
Inv. Status:
In Stock

CAT No:
HTS-T-027F
CAS No :
NA
Mol. Formula:
C19H38N6O10
Mol. Weight:
510.5
Inv. Status:
In Stock

CAT No:
HTS-F-014D
CAS No :
1262243-12-8
Mol. Formula:
C10H25NO11P2
Mol. Weight:
397.25
Inv. Status:
In Stock

CAT No:
HTS-L-008G
CAS No :
1356839-89-8
Mol. Formula:
C42H60N6O9
Mol. Weight:
792.98
Inv. Status:
In Stock

CAT No:
HTS-A-010AAD
CAS No :
186826-68-6
Mol. Formula:
C47H61NO4
Mol. Weight:
704.01
Inv. Status:
In Stock

CAT No:
HTS-A-010AD
CAS No :
186826-70-0
Mol. Formula:
C43H57NO2
Mol. Weight:
619.93
Inv. Status:
In Stock

CAT No:
HTS-F-017A
CAS No :
NA
Mol. Formula:
C17H18F6N2O2
Mol. Weight:
396.33
Inv. Status:
In Stock

CAT No:
HTS-S-001G
CAS No :
1391051-88-9
Mol. Formula:
C50H72N2O7
Mol. Weight:
813.13
Inv. Status:
In Stock

CAT No:
HTS-T-027F
CAS No :
NA
Mol. Formula:
C19H38N6O10
Mol. Weight:
510.5
Inv. Status:
In Stock

CAT No:
HTS-F-014D
CAS No :
1262243-12-8
Mol. Formula:
C10H25NO11P2
Mol. Weight:
397.25
Inv. Status:
In Stock

CAT No:
HTS-L-008G
CAS No :
1356839-89-8
Mol. Formula:
C42H60N6O9
Mol. Weight:
792.98
Inv. Status:
In Stock

CAT No:
HTS-A-010AAD
CAS No :
186826-68-6
Mol. Formula:
C47H61NO4
Mol. Weight:
704.01
Inv. Status:
In Stock

CAT No:
HTS-A-010AD
CAS No :
186826-70-0
Mol. Formula:
C43H57NO2
Mol. Weight:
619.93
Inv. Status:
In Stock

CAT No:
HTS-F-017A
CAS No :
NA
Mol. Formula:
C17H18F6N2O2
Mol. Weight:
396.33
Inv. Status:
In Stock

CAT No:
HTS-S-001G
CAS No :
1391051-88-9
Mol. Formula:
C50H72N2O7
Mol. Weight:
813.13
Inv. Status:
In Stock

CAT No:
HTS-T-027F
CAS No :
NA
Mol. Formula:
C19H38N6O10
Mol. Weight:
510.5
Inv. Status:
In Stock

CAT No:
HTS-F-014D
CAS No :
1262243-12-8
Mol. Formula:
C10H25NO11P2
Mol. Weight:
397.25
Inv. Status:
In Stock

CAT No:
HTS-L-008G
CAS No :
1356839-89-8
Mol. Formula:
C42H60N6O9
Mol. Weight:
792.98
Inv. Status:
In Stock

CAT No:
HTS-A-010AAD
CAS No :
186826-68-6
Mol. Formula:
C47H61NO4
Mol. Weight:
704.01
Inv. Status:
In Stock

CAT No:
HTS-A-010AD
CAS No :
186826-70-0
Mol. Formula:
C43H57NO2
Mol. Weight:
619.93
Inv. Status:
In Stock